Stanford: Ua hōʻemi mākou i ke kaumaha o nā pantograph lithium-ion ma 80 pakeneka. Hoʻonui ka nui o ka ikehu e 16-26 pakeneka.
Ua hoʻoholo nā kānaka ʻepekema ma ke Kulanui ʻo Stanford a me ka Stanford Linear Accelerator Center (SLAC) e hōʻemi i nā cell lithium-ion e hōʻemi i ko lākou kaumaha a pēlā e hoʻonui ai i ka nui o ka ikehu i mālama ʻia. No ka hana ʻana i kēia, hana hou lākou i nā papa haʻahaʻa haʻahaʻa i waho: ma kahi o nā ʻāpana ākea o ke keleawe a i ʻole alumini, ua hoʻohana lākou i nā ʻāpana metala liʻiliʻi, i hoʻohui ʻia me kahi papa polymer.
ʻOi aku ka nui o ka ikehu kiʻekiʻe ma ka Li-ion me ka ʻole o nā kumukūʻai hoʻopukapuka kiʻekiʻe
ʻO kēlā me kēia cell lithium-ion he ʻōwili i loko o kahi papa hoʻokuʻu / hoʻokuʻu, kahi electrode, electrolyte, electrode, a me kahi ʻohi o kēia manawa i kēlā kauoha. ʻO nā ʻaoʻao o waho he pahu metala i hana ʻia me ke keleawe a i ʻole alumini. Hāʻawi lākou i nā electrons e haʻalele a hoʻi i ke kelepona.
Ua hoʻoholo ka poʻe ʻepekema mai Stanford a me SLAC e nānā i nā mea ʻohi, no ka mea, ʻo ko lākou kaumaha he mau ʻumi o ka pakeneka o ke kaumaha o ka loulou holoʻokoʻa. Ma kahi o nā ʻāpana keleawe, hoʻohana lākou i nā kiʻiʻoniʻoni polymer me nā ʻāpana keleawe liʻiliʻi. Ua ʻike ʻia ua hiki ke hoʻemi i ke kaumaha o nā ʻohi a hiki i ka 80 pakeneka:
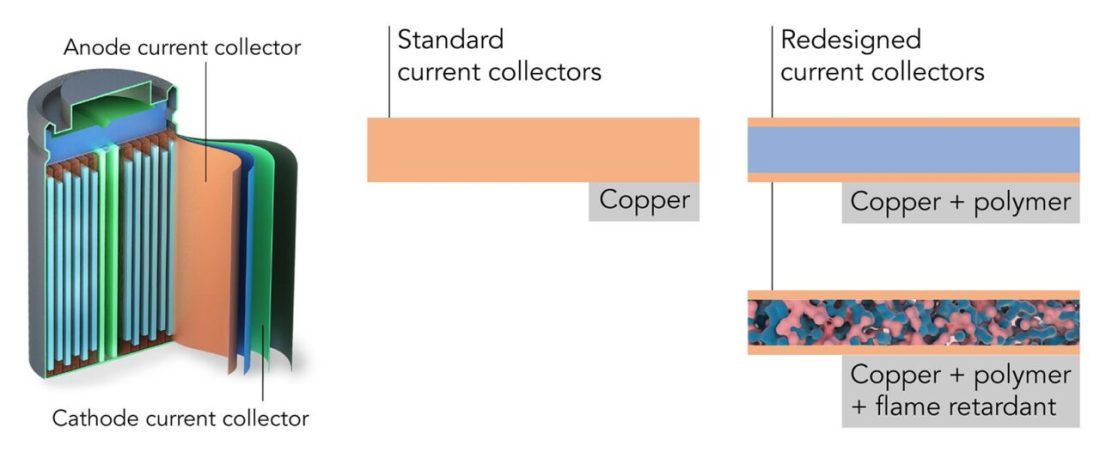
ʻO ke kelepona lithium-ion cylindrical maʻamau he ʻōwili lōʻihi i loaʻa i kekahi mau papa. Ua hōʻemi ka poʻe ʻepekema mai Stanford a me SLAC i nā papa e hōʻiliʻili a hoʻokō i nā hoʻopiʻi—nā ʻohi o kēia manawa. Ma kahi o nā ʻāpana keleawe, ua hoʻohana lākou i nā ʻāpana polymer-copper i hoʻonui ʻia me nā kemika hiki ʻole ke puhi ʻia (c) Yusheng E / Stanford University
ʻAʻole ʻo ia wale nō: hiki ke hoʻohui ʻia nā pūhui kemika i ka polymer e pale ai i ka ʻāʻī ʻana, a laila hele pū ka flammability haʻahaʻa o nā mea me kahi kaumaha haʻahaʻa:

ʻO ka flammability o ka pahu keleawe i hoʻohana ʻia i loko o kahi cell lithium-ion maʻamau a me kahi ʻohi i hoʻomohala ʻia e nā mea noiʻi ʻAmelika (c) Yusheng E / Stanford University
Wahi a nā mea noiʻi, hiki i nā mea hōʻiliʻili hana hou ke hoʻonui i ka nui o ka ikehu gravimetric o nā cell ma 16-26 pākēneka (= 16-26 pākēneka ʻoi aku ka ikehu no ka ʻāpana like o ka nuipa). ʻO ia hoʻi ʻO ka pākaukau o ka nui like a me ka nui o ka ikehu hiki ke 20 pakeneka ʻoi aku ka māmā ma mua o kēia manawa.
Ua hoʻāʻo ʻia i ka wā ma mua e hoʻomaikaʻi i ka waihona, akā ʻo ka hoʻololi ʻana iā lākou ua alakaʻi i nā hopena ʻaoʻao i manaʻo ʻole ʻia. Ua paʻa ʻole nā pūnaewele a ʻoi aku ka nui o ka electrolyte. ʻAʻole ʻike ʻia ka ʻano like ʻole i hoʻomohala ʻia e nā ʻepekema ma Stanford i nā pilikia.
Aia kēia mau hoʻomaikaʻi i ka noiʻi mua ʻana, no laila, mai manaʻo e paʻi lākou i ka mākeke ma mua o 2023. Eia naʻe, ke nānā aku nei lākou he mea hoʻohiki.
Pono e hoʻohui ʻia he manaʻo hoihoi ʻo Tesla e hōʻiliʻili i ka uku o nā papa metala. Ma kahi o ka hoʻohana ʻana i nā ʻāpana keleawe lahilahi ma ka lōʻihi holoʻokoʻa o ka ʻōwili a lawe ʻia i waho ma kahi wale nō (ma waena), lawe koke ia i waho me ka hoʻohana ʻana i ka ʻoki ʻoki ʻia. ʻO kēia ka mea e neʻe ai nā uku i kahi mamao liʻiliʻi (kū'ē!), A hāʻawi ke keleawe i ka hoʻoili wela hou i waho:
> E hoʻoluʻu ʻia nā kelepona 4680 i loko o nā pihi hou o Tesla mai luna a lalo? Mai lalo wale nō?
E hoihoi paha kēia iā ʻoe:
