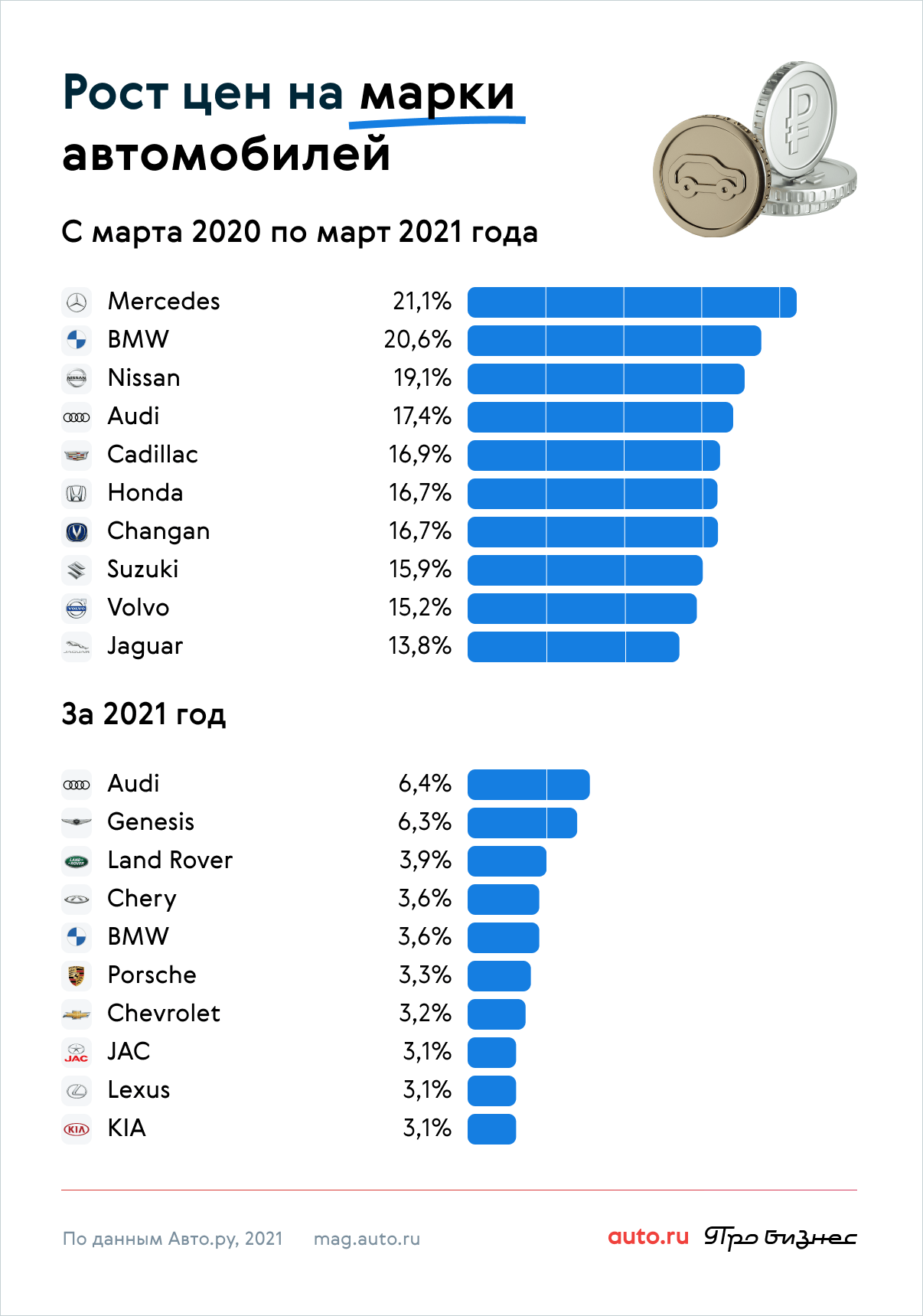
Kohu wai
ʻO ke aniani wai he hopena koʻikoʻi o ka sodium metasilicate Na2SiO3 (hoʻohana pū ʻia ka paʻakai potassium). Hana ʻia ia ma ka hoʻoheheʻe ʻana i ka silica (e like me ke one) i loko o kahi solution sodium hydroxide:
Kohu wai ʻoiaʻiʻo, he hui ia o nā paʻakai o nā ʻakika silicic like ʻole me nā degere like ʻole o ka polymerization. Hoʻohana ʻia ia ma ke ʻano he impregnation (no ka laʻana, e pale i nā paia mai ka makū, ma ke ʻano he pale ahi), kahi ʻāpana o nā mea hoʻopihapiha a me nā mea hoʻopaʻa, no ka hana ʻana i nā mea silicone, a me kahi meaʻai meaʻai e pale ai i ka caking (E 550). Hiki ke hoʻohana ʻia ke aniani wai kūʻai no nā hoʻokolohua kupaianaha (no ka mea he wai mānoanoa, syrupy, hoʻohana ʻia ia ma ka hoʻoheheʻe ʻana me ka wai ma ka ratio o 1:1).
I ka hoʻokolohua mua, e hoʻoheheʻe mākou i kahi hui o nā waikawa silicic. No ka ho'āʻo, e hoʻohana mākou i kēia mau hopena: ke aniani wai a me ka ammonium chloride NH.4Cl a me ka pepa kuhikuhi e nānā i ka hopena (kiʻi 1).
Kemika – ʻāpana o ke aniani wai 1 – MT
ʻO ke aniani wai e like me ka paʻakai o ka waika nāwaliwali a me kahi kumu ikaika i loko o kahi wai wai ka nui o ka hydrolyzed a he alkaline (kiʻi 2). E ninini i ka solution ammonium chloride (kiʻi 3) i loko o ka beaker me ka solution aniani wai a hoʻoulu i nā mea i loko (photo 4). Ma hope o kekahi manawa, ua hoʻokumu ʻia kahi papa gelatinous (kiʻi 5), ʻo ia ka hui ʻana o nā waikawa silicic:
(ʻoiaʻiʻo SiO2?nGn2O ? ua hoʻokumu ʻia nā ʻakika silicic me nā pae like ʻole o ka hydration).
ʻO ke ʻano hana hoʻoheheʻe beaker i hōʻike ʻia e ka hoʻohālikelike hōʻuluʻulu ma luna nei penei:
a) ʻo ka sodium metasilicate i ka hoʻonā i hoʻokaʻawale ʻia a komo i ka hydrolysis:
b) hoʻopili nā ion ammonium me nā ion hydroxide:
I ka pau ʻana o nā ion hydroxyl i ka pane b), ua neʻe ka equilibrium o ka pane ʻana a) i ka ʻākau a ma muli o ka hopena, ua hoʻoheheʻe ʻia nā ʻakika silicic.
Ma ka lua o ka hoʻokolohua, ulu mākou i nā "mea kanu kemika". E koi ʻia nā haʻina aʻe no ka hoʻokolohua: aniani wai a me nā paʻakai metala? hao (III), hao (II), keleawe (II), calcium, tin (II), chromium (III), manganese (II).
Kemika – ʻāpana o ke aniani wai 2 – MT
E hoʻomaka kākou i ka hoʻokolohua ma ka hoʻokomo ʻana i kekahi mau kristal o ka hao chloride (III) paʻakai FeCl i loko o kahi paipu hoʻāʻo.3 a me ka hopena o ke aniani wai (kiʻi 6). Ma hope o kekahi manawa, ʻeleʻele? (kiʻi 7, 8, 9), mai ka hao insoluble (III) metasilicate:
Eia kekahi, hiki i nā paʻakai o nā metala ʻē aʻe ke loaʻa nā hopena maikaʻi:
- keleawe(II)? kiʻi 10
- chromium(III)? kiʻi 11
- hao(II)? kiʻi 12
- calcium? kiʻi 13
- manganese(II)? kiʻi 14
- alakai(II)? kiʻi 15
Hoʻokumu ʻia ke ʻano o nā kaʻina hana e pili ana i ke ʻano o ka osmosis, ʻo ia hoʻi, ke komo ʻana o nā mea liʻiliʻi ma o nā pores o nā membrane semipermeable. ʻO nā waihona o nā silicates metala hiki ʻole ke hoʻoheheʻe ʻia ma ke ʻano he ʻāpana lahilahi ma ka ʻili o ka paʻakai i hoʻokomo ʻia i loko o ka paipu hoʻāʻo. Komo nā mole wai i loko o nā pores o ka membrane hopena, e hoʻoheheʻe ai ka paʻakai metala ma lalo. ʻO ka hopena hopena e hoʻokuʻu i ke kiʻiʻoniʻoni a hiki i ka pohā. Ma hope o ka ninini ʻana i ka wai hoʻoheheʻe paʻakai metala, e hoʻoheheʻe hou ʻia ka wai silicate? hoʻi hou ka pōʻai iā ia iho a me ka mea kanu kemika? mahuahua.
Ma ka waiho ʻana i ka hui ʻana o nā kristal paʻakai o nā metala like ʻole i loko o ka ipu hoʻokahi a me ka hoʻoinu ʻana me kahi hopena o ke aniani wai, hiki iā mākou ke ulu i kahi "māla kemika" holoʻokoʻa? (kiʻi 16, 17, 18).
Nā kiʻi