Ka hana ʻana i nā kumu ikehu kemika
ʻO kahi kūlana maʻamau i kēlā me kēia hale, ʻaʻole maikaʻi nā pākaukau i kūʻai ʻia i kēia manawa. A i ʻole, ʻo ka mālama ʻana i ke kaiapuni, a i ka manawa like - e pili ana i ka waiwai o kā mākou ʻeke kālā, loaʻa iā mākou nā pā? Ma hope o kekahi manawa, hōʻole lākou e hui pū. No laila i ka ʻōpala? ʻAʻole loa! I ka ʻike e pili ana i nā hoʻoweliweli a nā cell e hana ai i ke kaiapuni, e ʻimi mākou i kahi hui.
ʻO ka hōʻiliʻili
He aha ka nui o ka pilikia a mākou e hana nei? Ua hōʻike ʻia kahi hōʻike 2011 a ka Luna Hoʻokele Kaiapuni Nui ma mua o ʻO 400 miliona mau pūnaewele a me nā pā. Ma kahi o ka helu like i pepehi iā lākou iho.
Laiki. 1. Awelika haku mele 'ana o nā mea maka (nā pūnaewele i ho'ohana 'ia) mai nā hō'ili'ili moku'āina.
No laila pono mākou e hoʻomohala ma kahi o 92 tausani tons o ka ʻōpala pōʻino loaʻa nā metala kaumaha (mercury, cadmium, nickel, silver, lead) a me ka helu o nā pūhui kemika (potassium hydroxide, ammonium chloride, manganese dioxide, sulfuric acid) (Fig. 1). Ke kiola mākou iā lākou - ma hope o ka ʻino ʻana o ka uhi - hoʻohaumia lākou i ka lepo a me ka wai (Fig. 2). Mai hana mākou i "makana" i ke kaiapuni, a no laila iā mākou iho. ʻO kēia nui, ʻo 34% i helu ʻia e nā mea hana kūikawā. No laila, he nui nā mea e hana ʻia, ʻaʻole ia he hōʻoluʻolu ʻaʻole ma Polani wale nō?
Laiki. 2. ʻO nā ʻāpana cell corroded.
ʻAʻohe o mākou kumu e hele ai nā pūnaewele hoʻohana. ʻO kēlā me kēia puka e kūʻai aku ana i nā pila a me nā mea pani e koi ʻia e ʻae iā lākou mai iā mākou (me nā mea uila kahiko a me nā mea hana hale). Eia kekahi, nui nā hale kūʻai a me nā kula i nā ipu i hiki ai iā mākou ke waiho i nā hīnaʻi. No laila, ʻaʻole mākou e "hoʻokuʻu" a ʻaʻole e hoʻolei aku i nā ʻōpala i hoʻohana ʻia a me nā accumulators i ka ʻōpala. Me ka makemake liʻiliʻi, e ʻike mākou i kahi hōʻuluʻulu hui, a ʻo nā loulou ponoʻī ke kaumaha liʻiliʻi i ʻole e luhi ka loulou iā mākou.
Kōkua
E like me nā mea ʻē aʻe mea hiki ke hana hou ia, ʻo ka hoʻololi maikaʻi ʻana ma hope o ka hoʻokaʻawale ʻana. ʻO ka ʻōpala mai nā mea kanu ʻoihana ʻano like ʻole i ka maikaʻi, akā ʻo ka ʻōpala mai nā hōʻiliʻili lehulehu he hui pū ʻia o nā ʻano kelepona i loaʻa. No laila, lilo ka nīnau nui kaawale.
Ma Polani, ua hana lima 'ia ka ho'oka'awale 'ana, a'o nā 'āina 'ē a'e o 'Eulopa ua loa'a i nā laina ho'oka'awale 'akomi. Hoʻohana lākou i nā kānana me nā nui mesh kūpono (ʻae ka hoʻokaʻawale ʻana o nā ʻāpana like ʻole) a me ka x-ray (ka hoʻokaʻina maʻiʻo). He ʻokoʻa iki ke ʻano o nā mea maka mai nā hōʻiliʻili ma Polani.
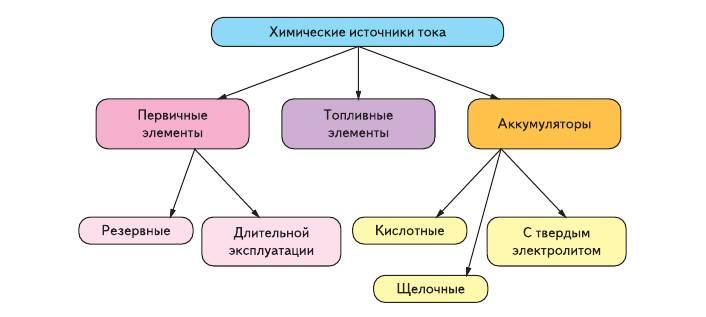
A hiki i kēia manawa, ua lanakila kā mākou mau cell acidic Leclanche. ʻO kēia wale nō ka mea i ʻike ʻia ka maikaʻi o nā mea alkaline hou, i lanakila ai i nā mākeke Komohana i nā makahiki he nui aku nei. ʻO kēlā me kēia hihia, ʻoi aku nā ʻano ʻelua o nā cell disposable i ʻoi aku ma mua o 90% o nā pākahi i hōʻiliʻili ʻia. ʻO ke koena, he pihi pihi (nā wati mana (Fig. 3) a i ʻole nā calculators), nā ʻaʻa rechargeable a me nā pā lithium no nā kelepona a me nā kamepiula. ʻO ke kumu o ia ʻāpana liʻiliʻi ʻo ke kumukūʻai kiʻekiʻe a me ke ola lawelawe ʻoi aku ka lōʻihi o ka hoʻohālikelike ʻana i nā mea hoʻopau.
Laiki. 3. Hoʻohana ʻia ka loulou kala no ka hoʻoikaika ʻana i nā wati lima.
Hoʻomākaukau
Ma hope o ka haʻihaʻi ʻana, ʻo ia ka manawa no ka mea nui loa kaʻina hana - ka hoʻihoʻi ʻana i nā mea maka. No kēlā me kēia ʻano, ʻokoʻa iki nā huahana i loaʻa. Eia nō naʻe, ʻano like nā ʻenehana hana.
mīkini hana aia i ka wili ʻana i nā ʻōpala ma nā wili. Hoʻokaʻawale ʻia nā ʻāpana i loaʻa me ka hoʻohana ʻana i nā electromagnets (hao a me kāna mau alloys) a me nā ʻōnaehana sieve kūikawā (nā metala ʻē aʻe, nā mea plastic, pepa, etc.). Zaleto Aia ke ala i ka ʻoiaʻiʻo ʻaʻole pono e hoʻokaʻawale pono i nā mea maka ma mua o ka hana ʻana, kīnā - ka nui o nā ʻōpala hiki ʻole ke hoʻohana ʻia e pono ke hoʻolei ʻia ma nā ʻōpala.
ʻO ka hana hou ʻana hydrometallurgical ʻo ia ka hoʻoheheʻe ʻana o nā cell i loko o nā ʻakika a i ʻole nā kumu. I ka pae aʻe o ka hana ʻana, hoʻomaʻemaʻe ʻia nā hopena hopena a hoʻokaʻawale ʻia, no ka laʻana, nā paʻakai metala, e loaʻa ai nā mea maʻemaʻe. Nui pōmaikaʻi ʻike ʻia ke ala e ka hoʻohana ʻana i ka ikehu haʻahaʻa a me kahi ʻōpala liʻiliʻi e pono ai ke hoʻolei. Kīnā ʻole Pono kēia ʻano hana hou e hoʻokaʻawale pono i nā pila i mea e pale aku ai i ka haumia o nā huahana.
Ka hana wela aia i ka puhi ana i na keena i loko o na kapuahi o ka manao kupono. ʻO ka hopena, hoʻoheheʻe ʻia kā lākou oxides a loaʻa (nā mea maka no nā wili kila). Zaleto Aia ke ala i ka hiki ke hoʻohana i nā pākahi i hoʻokaʻawale ʻole ʻia, kīnā a - ka hoʻohana ʻana i ka ikehu a me ka hana ʻana i nā huahana hoʻopau ʻino.
Ma waho aʻe hiki ke hana hou Hoʻopaʻa ʻia nā cell i loko o nā pae ʻāina ma hope o ka pale mua ʻana i ke komo ʻana o kā lākou mau mea i loko o ke kaiapuni. Eia nō naʻe, he hapa wale nō kēia, e hoʻopaneʻe ana i ka pono e hoʻoponopono i kēia ʻano ʻōpala a me ka ʻōpala o nā waiwai maka waiwai.
Hiki iā mākou ke hoʻihoʻi hou i kekahi o nā meaʻai i loko o kā mākou lab home. ʻO kēia nā ʻāpana o nā mea Leclanche maʻamau - ka zinc maʻemaʻe kiʻekiʻe mai nā kīʻaha e hoʻopuni ana i ka mea, a me nā electrodes graphite. ʻO kahi ʻē aʻe, hiki iā mākou ke hoʻokaʻawale i ka manganese dioxide mai ka hui ʻana i loko o ka hui ʻana - e hoʻolapalapa wale iā ia me ka wai (e wehe i nā haumia hiki ke hoʻoheheʻe ʻia, ka nui o ka ammonium chloride) a kānana. ʻO ke koena hiki ʻole ke hoʻoheheʻe ʻia (i hoʻohaumia ʻia me ka lepo lanahu) kūpono no ka hapa nui o nā hopena e pili ana i ka MnO.2.
Akā ʻaʻole hiki ke hoʻohana hou ʻia nā mea i hoʻohana ʻia e hoʻohana i nā mea hana hale. ʻO nā pila kaʻa kahiko kekahi kumu o nā mea maka. Lawe ʻia ke alakaʻi mai ia mau mea, a laila hoʻohana ʻia i ka hana ʻana i nā mea hou, a hoʻopau ʻia nā hihia a me ka electrolyte hoʻopiha iā lākou.
ʻAʻohe mea pono e hoʻomanaʻo ʻia i ka pōʻino o ke kaiapuni i hiki ke hoʻoulu ʻia e ka metala kaumaha a me ka solution sulfuric acid. No ko mākou hoʻomohala wikiwiki ʻana i ka moʻomeheu ʻenehana, he kumu hoʻohālike ka laʻana o nā cell a me nā pākahi. ʻO ka pilikia nui aʻe ʻaʻole ka hana ʻana o ka huahana ponoʻī, akā ʻo kona hoʻopau ʻana ma hope o ka hoʻohana ʻana. Manaʻo wau e hoʻoikaika ka poʻe heluhelu o ka "Young Technician" i nā poʻe ʻē aʻe e hana hou i kā lākou hiʻohiʻona.
Hoʻāʻo 1 - pākaukau lithium
nā pūnaewele lithium hoʻohana ʻia lākou i nā calculators a no ka mālama ʻana i ka mana i ka BIOS o nā motherboards kamepiula (Fig. 4). E hōʻoia mākou i ka hele ʻana o ka lithium metala i loko o lākou.
Laiki. 4. ʻO kahi kelepona lithium-manganese i hoʻohana ʻia e mālama i ka mana i ka BIOS o kahi motherboard computer.
Ma hope o ka hoʻokaʻawale ʻana i ka mea (no ka laʻana, ke ʻano maʻamau CR2032), hiki iā mākou ke ʻike i nā kikoʻī o ke ʻano (Fig. 5): ʻeleʻele ʻeleʻele ʻeleʻele o ka manganese dioxide MnO2, he electrode hoʻokaʻawale porous i hoʻokomo ʻia me kahi solution electrolyte organic, e hoʻokaʻawale ana i ke apo plastik a me ʻelua mau ʻāpana metala e hana ana i hale.
Laiki. 5. Nā ʻāpana o kahi cell lithium-manganese: 1. ʻO ka ʻāpana haʻahaʻa o ke kino me kahi papa o ka metala lithium (negative electrode). 2. Hoʻopili ʻia ka mea hoʻokaʻawale me kahi hopena electrolyte organik. 3. Paʻi paʻi o ka manganese dioxide (positive electrode). 4. Ke apo plastik (electrode insulator). 5. Hale luna (positive electrode terminal).
ʻO ka mea liʻiliʻi (ʻo ka electrode maikaʻi ʻole) ua uhi ʻia me kahi papa o ka lithium, e pōʻeleʻele koke i ka ea. ʻIke ʻia ka mea e ka hoʻāʻo ahi. No ka hana ʻana i kēia, e lawe i kekahi metala palupalu ma ka hope o ka uea hao a hoʻokomo i ka hāpana i loko o ka lapalapa ahi - ʻo ke kala carmine e hōʻike ana i ka hele ʻana o ka lithium (Fig. 6). Hoʻopau mākou i nā koena metala ma ka hoʻoheheʻe ʻana iā lākou i ka wai.
Laiki. 6. He laʻana o ka lithium i loko o ka lapalapa ahi.
E kau i ka electrode metala me kahi papa lithium i loko o ka beaker a ninini i kekahi mau kenimika3 wai. Hiki mai ka hopena ikaika i loko o ka moku, me ka hoʻokuʻu ʻana o ke kinoea hydrogen:
He kumu ikaika ka Lithium hydroxide a hiki iā mākou ke hoʻāʻo maʻalahi me ka pepa kuhikuhi.
ʻIke 2 - ka pilina alkaline
E ʻoki i kahi mea alkaline hiki ke hoʻohana ʻia, no ka laʻana, ʻano LR6 ("manamana", AA). Ma hope o ka wehe ʻana i ke kīʻaha metala, ʻike ʻia ke ʻano o loko (Fig. 7): aia i loko kahi ʻāpana māmā e hana ana i kahi anode (potassium a i ʻole sodium hydroxide a me ka lepo zinc), a me kahi papa ʻeleʻele o ka manganese dioxide MnO a puni ia.2 me ka lepo graphite (cell cathode).
Laiki. 7. ʻO ka hopena alkaline o ka anode nuipa i loko o ke kelepona alkaline. ʻIke ʻia ke ʻano kelepona: ʻanode-forming mass māmā (KOH + zinc dust) a me ka manganese dioxide ʻeleʻele me ka lepo graphite ma ke ʻano he cathode.
Hoʻokaʻawale ʻia nā electrodes mai kekahi i kekahi e kahi diaphragm pepa. E hoʻopili i kahi liʻiliʻi o ka mea māmā i ka pahu hoʻāʻo a hoʻomāmā iā ia me kahi kulu wai. Hōʻike ke kala uliuli i ka hopena alkaline o ka anode nuipa. ʻO ke ʻano o ka hydroxide i hoʻohana ʻia e hōʻoia maikaʻi ʻia e ka hoʻāʻo ahi. Hoʻopili ʻia kahi laʻana e like me ka nui o nā ʻanoʻano poppy i kahi uwea hao i pulu i ka wai a waiho ʻia i loko o kahi lapalapa ahi.
Hōʻike ka waihoʻoluʻu melemele i ka hoʻohana ʻana o ka sodium hydroxide e ka mea hana, a ʻo ka ʻulaʻula-ʻulaʻula e hōʻike ana i ka potassium hydroxide. Ma muli o ka hoʻohaumia ʻana o nā pūhui sodium i nā mea a pau, a ʻo ka hoʻāʻo ʻana o ka lapalapa no kēia ʻano mea koʻikoʻi loa, hiki i ka waihoʻoluʻu melemele o ka lapalapa ke uhi i nā laina spectral o ka potassium. ʻO ka hopena e nānā i ka lapalapa ma o kahi kānana polū-violet, hiki ke lilo i ke aniani cobalt a i ʻole ka wai kala i loko o ka ipu (indigo a i ʻole methyl violet i loaʻa i ka disinfectant ʻeha, pyoctane). Hoʻopili ka kānana i ka waihoʻoluʻu melemele, e ʻae iā ʻoe e hōʻoia i ka hele ʻana o ka potassium i ka hāpana.
Nā code koho
No ka hoʻomaʻamaʻa ʻana i ke ʻano kelepona, ua hoʻokomo ʻia kahi code alphanumeric kūikawā. No nā ʻano maʻamau i loko o ko mākou mau home, ʻano like ia: helu-hua-hua-hua, kahi:
- ʻo ka huahelu mua ka helu o nā cell; mālama ʻole ʻia no nā cell hoʻokahi.
– hōʻike ka leka mua i ke ʻano kelepona. Ke haʻalele ʻia, he Leclanche zinc-graphite cell (anode: zinc, electrolyte: ammonium chloride, NH4Cl, zinc chloride ZnCl2, cathode: manganese dioxide MnO2). Ua kapa inoa ʻia nā ʻano cell ʻē aʻe penei (ua hoʻohana pū ʻia ka sodium hydroxide ma kahi o ka potassium hydroxide):
A, P - nā mea zinc-air (anode: zinc, hoʻemi ʻia ka oxygen atmospheric ma kahi cathode graphite);
B, C, E, F, G - nā pūnaewele lithium (anode: lithium, akā nui nā mea i hoʻohana ʻia e like me nā cathodes a me nā electrolyte);
H – Ni-MH nickel-metal hydride pākahiko (metal hydride, KOH, NiOOH);
K – Ni-Cd nickel-cadmium pākahi (cadmium, KOH, NiOOH);
L - ʻeleʻele alkaline (zinc, KOH, MnO2);
M - ʻeleʻele mercury (zinc, KOH; HgO), ʻaʻole hoʻohana hou ʻia;
S - mea kala (zinc, KOH; Ag2E pili ana);
Z - ʻeleʻele nickel-manganese (zinc, KOH, NiOOH, MnO2).
- e hōʻike ana kēia leka i ke ʻano o ka loulou:
F - lamellar;
R - cylindrical;
S - huinahā;
P - ke koho ʻana i kēia manawa o nā cell me nā ʻano ʻē aʻe ma waho o ka cylindrical.
- ʻo ka helu hope a i ʻole nā kiʻi e hōʻike i ka nui o ka kuhikuhi (nā helu helu a hāʻawi pololei i nā ana).
Ka helu ʻana i nā laʻana:
R03
- he pulina zinc-graphite ka nui o ka manamana lima. ʻO kekahi inoa ʻo AAA a i ʻole micro.
LR6 - he cell alkaline ka nui o ka manamana lima. ʻO kekahi inoa ʻē aʻe ʻo AA a i ʻole minion.
HR14 - ʻO ka pākaukau Ni-MH, hoʻohana pū ʻia ka leka C no ka nui.
KR20 - ʻO ka pākaukau Ni-Cd, ka nui o ia mea i kaha ʻia me ka leka D.
3LR12 - he pākaukau palahalaha me ka volta o 4,5 V, i loko o ʻekolu mau ʻāpana alkaline.
6F22 – 9V pākaukau; ua hoʻopaʻa ʻia i loko o kahi pahu pahu ʻehā.
CR2032 - ke kelepona lithium-manganese (lithium, organik electrolyte, MnO2) me ke anawaena o 20 mm a me ka mānoanoa o 3,2 mm.
