Loaʻa ka holomua ma ka ʻenehana pākaukau Li-S: ma luna o 99%. mana ma hope o 200 cycles
Ua hoʻolaha ka poʻe ʻepekema ma ke Kulanui o Melbourne (Australia) i ka holomua o ka ʻenehana hoʻopaʻa ʻana i ka pā lithium-sulfur (Li-S). Ua hiki iā lākou ke hana i nā pūnaewele i mālama ʻia ma mua o 99 ka nui o ko lākou hiki ma hope o 200 cycles o ka hana a hāʻawi i nā manawa he nui i ka mana o nā cell lithium-ion no ke kaumaha like.
Nā mea Li-S - aia nā pilikia, aia nā hoʻonā
ʻAʻole hou ka manaʻo o ka hoʻohana ʻana i ka sulfur i loko o nā cell: Ua hoʻohana mua ʻia nā pā Li-S i ka makahiki 2008 ma ka Zephyr-6, ka mea i uhaʻi i ka moʻolelo no ka pae pae ʻole. Hiki iā ia ke hoʻomau i ka lewa no kahi kokoke i 3,5 mau lā me ka māmā o nā ʻeke lithium-sulfur i hoʻoikaika i ka ʻenekini a hoʻopaʻa iā lākou iho mai nā pihi photovoltaic (kumu).
Eia naʻe, loaʻa i nā pūnaewele Li-S hoʻokahi hemahema nui: kū i nā ʻumi o nā pōʻai hanaNo ka mea, i ka wā e hoʻopiʻi ai, hoʻonui ka cathode i ka sulfur i kona leo ma kahi o 78 pakeneka (!), ʻO ia ka 8 mau manawa ma mua o ka graphite i loko o nā keena lithium-ion. ʻO ka pehu ʻana o ka cathode e hoʻoheheʻe ʻia a hoʻoheheʻe i ka sulfur i loko o ka electrolyte.
A ʻo ka liʻiliʻi o ka nui o ka cathode, ʻoi aku ka liʻiliʻi o ka hiki o ke kelepona holoʻokoʻa - hiki koke ka degradation.
> Pehea ka lōʻihi o ke kaʻa uila? ʻEhia makahiki e pani ai ka pilahi uila? [E PANE MAKOU]
Ua hoʻoholo ka poʻe ʻepekema ʻo Melbourne e hoʻopili i nā molekele sulfur me kahi polymer, akā hāʻawi iā lākou i kahi wahi liʻiliʻi ma mua o ka wā ma mua. Ua hoʻololi ʻia kahi ʻāpana o nā paʻa paʻa e nā alahaka polymer maʻalahi, i hiki ai ke hoʻokō i kahi kūʻē kiʻekiʻe i ka luku me ka hoʻololi ʻana i ka leo - hoʻopili nā alahaka i nā mea cathode e like me ka rubber:
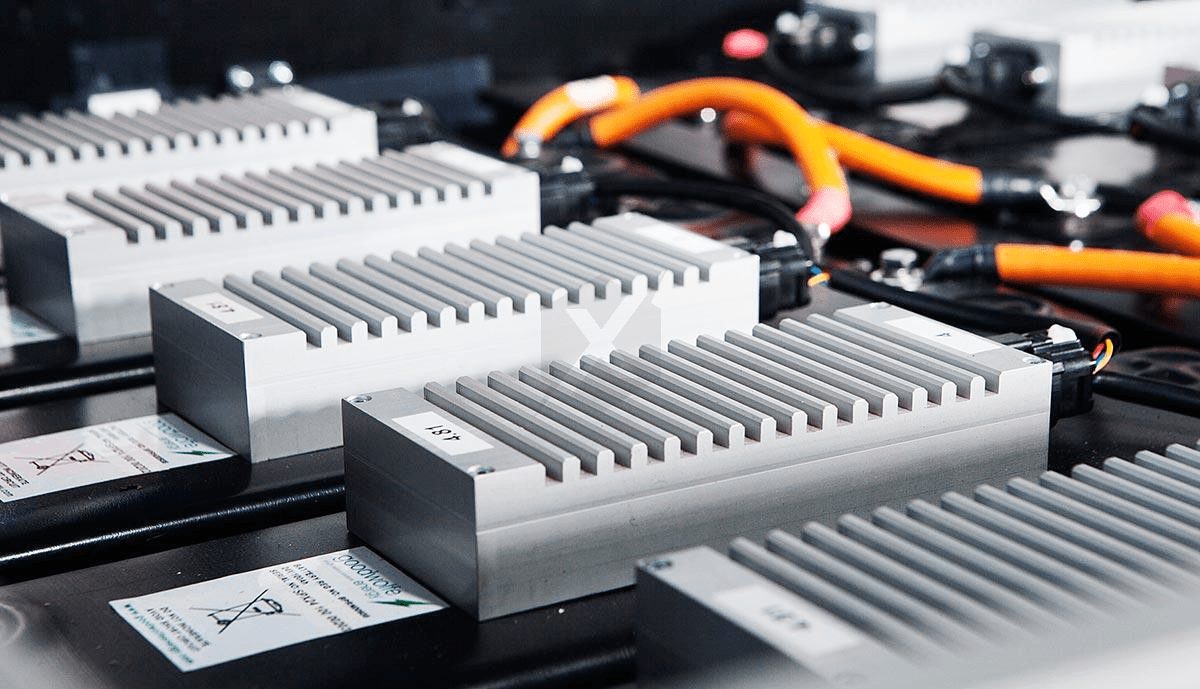
ʻO nā alahaka polymer e hoʻopili ana i nā hale o nā molekele sulfur (c) University of Melbourne
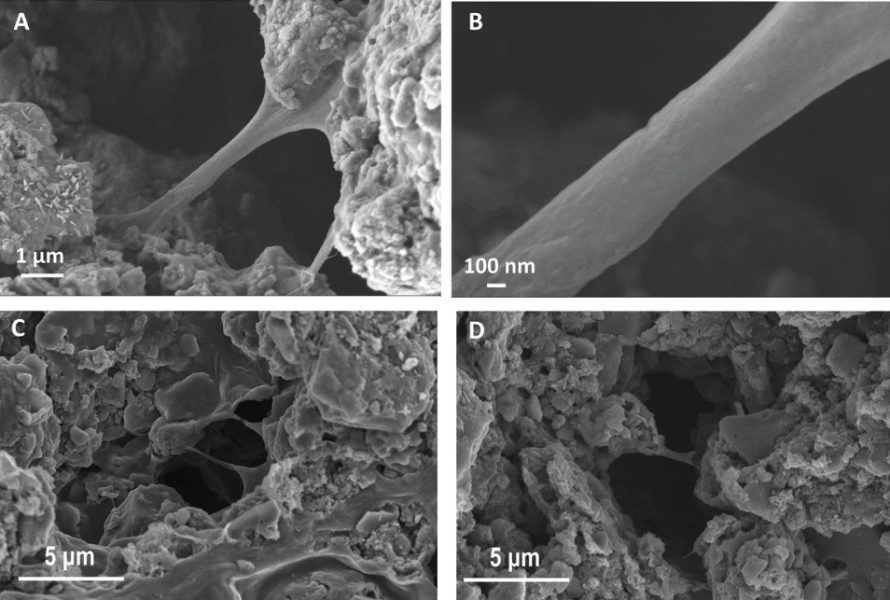
ʻO nā pūnaewele me nā cathodes i hoʻomaikaʻi ʻia i ko lākou maikaʻi loa. hiki iā lākou ke mālama i ka 99 pākēneka o ko lākou mana mua ma hope o 200 mau manawa hoʻoiho (kahi kumu). A ua mālama lākou i ka pono nui loa o ka sulfur: mālama lākou i 5 mau manawa ʻoi aku ka nui o ka ikehu ma mua o nā cell lithium-ion.
Nā mea liʻiliʻi? Lawe ʻia ka hoʻouka ʻana a me ka hoʻokuʻu ʻana ma ka mana o 0,1 C (0,1 x kaha), ma hope o 200 mau pōʻai, ʻo nā hāʻina maikaʻi loa i hāʻule i ka 80 pakeneka o ko lākou hiki mua... Eia kekahi, ma nā ukana kiʻekiʻe (ka hoʻouka ʻana / hoʻokuʻu ʻana ma 0,5 C), ua nalowale nā pūnaewele i ka 20 pakeneka o ko lākou hiki ma hope o kekahi mau kakini, a hiki i ka lōʻihi ma mua o 100 mau manawa.
Kiʻi wehe: Oxis lithium-sulfur cell, ka mea e manaʻo e hoʻolaha i kēia ʻenehana. Kiʻi hoʻohālike
E hoihoi paha kēia iā ʻoe:
