Ma mua o ka hana ʻekolu, ʻo ia hoʻi, e pili ana i ka loaʻa ʻana o ka radioactivity artificial
Mai kēlā manawa kēia manawa i ka mōʻaukala o ka physics aia nā makahiki "kamahaʻo" i ka wā i alakaʻi ʻia ai ka hui pū ʻana o nā mea noiʻi he nui i kahi ʻano o nā ʻike holomua. Pela no ka makahiki 1820, ka makahiki o ka uila, 1905, ka makahiki kupanaha o na pepa eha a Einstein, 1913, ka makahiki e pili ana i ke ao ana i ke ano o ka atom, a me ka hope 1932, i ka wa i ikeia ai ka ike loea a me ka holomua i ka ka hoʻokumu ʻana i ka physics nuklea.
male hou
ʻO Irina, ke kaikamahine hiapo a Marie Skłodowska-Curie a me Pierre Curie, ua hānau ʻia ma Paris ma 1897 (1). A hiki i ka makahiki ʻumikūmālua, ua hānai ʻia ʻo ia ma ka home, i loko o kahi "kula" liʻiliʻi i hana ʻia e nā ʻepekema kaulana no kāna mau keiki, aia ma kahi o ʻumi mau haumāna. ʻO nā kumu: ʻo Marie Sklodowska-Curie (physics), Paul Langevin (mathematics), Jean Perrin (chemistry), a ʻo ke kanaka i aʻo nui ʻia e nā makuahine o nā haumāna. Hana ʻia nā haʻawina ma nā hale o nā kumu, aʻo nā keiki e aʻo i ka physics a me ke kemika ma nā hale hana maoli.
No laila, ʻo ke aʻo ʻana i ka physics a me ke kemika ka loaʻa ʻana o ka ʻike ma o nā hana hoʻokō. Ua hauʻoli kēlā me kēia hoʻokolohua kūleʻa i nā mea noiʻi ʻōpio. He mau hoʻokolohua maoli kēia i pono e hoʻomaopopo ʻia a mālama pono ʻia, a ʻo nā keiki i loko o ka hale hana ʻo Marie Curie e pono i ke ʻano hoʻohālike. Pono nō e loaʻa ka ʻike theoretical. ʻO ke ʻano, e like me ka hopena o nā haumāna o kēia kula, ma hope o nā ʻepekema maikaʻi a koʻikoʻi, ua ʻike maikaʻi ʻia.
2. ʻO Frederic Joliot (kiʻi na Harcourt)
Eia kekahi, ʻo ke kupuna kāne o Irena, he kauka, ua hāʻawi nui i ka manawa i ka moʻopuna makua ʻole a kona makuakāne, e leʻaleʻa a hoʻonui i kāna hoʻonaʻauao ʻepekema kūlohelohe. I ka makahiki 1914, ua puka 'o Irene mai ka paionia Collège Sévigné a komo i ke kula o ka makemakika a me ka 'epekema ma Sorbonne. Ua like kēia me ka hoʻomaka ʻana o ke Kaua Honua Mua. I ka makahiki 1916 ua hui pū ʻo ia me kona makuahine a ua hoʻonohonoho pū lāua i kahi lawelawe radiological ma ke Keʻa ʻulaʻula Farani. Ma hope o ke kaua, ua loaʻa iā ia ke kēkelē laepua. I ka makahiki 1921, ua paʻi ʻia kāna hana ʻepekema mua. Ua kūpaʻa ʻo ia i ka hoʻoholo ʻana i ka nuipa atomika o ka chlorine mai nā minerala like ʻole. Ma kāna mau hana hou aku, ua hana pū ʻo ia me kona makuahine, e pili ana i ka radioactivity. Ma kāna palapala kauka kauka, pale ʻia i ka makahiki 1925, ua aʻo ʻo ia i nā ʻāpana alpha i hoʻokuʻu ʻia e ka polonium.
Frederic Joliot hānau ʻia i ka makahiki 1900 ma Paris (2). Mai kona mau makahiki ʻewalu i hele ai ʻo ia i ke kula ma So, noho ʻo ia ma ke kula hānai. I kēlā manawa, makemake ʻo ia i nā haʻuki ma mua o ke aʻo ʻana, ʻoi aku ka pôpeku. A laila ua huli ʻo ia i ʻelua kula kiʻekiʻe. E like me Irene Curie, ua hala koke kona makuakāne. I ka makahiki 1919, ua hala ʻo ia i ka hoʻokolokolo ma ka École de Physique et de Chemie Industrielle de la Ville de Paris (Ke Kula ʻOihana Physics a me Industrial Chemistry o ke kūlanakauhale ʻo Paris). Ua puka 'o ia i ka makahiki 1923. Ua aʻo kāna kaukaʻi ʻo Paul Langevin i nā mana a me nā pono o Frederick. Ma hope o 15 mau mahina o ka lawelawe kaua, ma ke kauoha a Langevin, ua koho ʻia ʻo ia i mea kōkua pilikino pilikino iā Marie Skłodowska-Curie ma ka Radium Institute me kahi haʻawina mai ka Rockefeller Foundation. Ma laila ʻo ia i hui ai me Irene Curie, a i ka makahiki 1926 ua male ka poʻe ʻōpio.
Ua hoʻopau ʻo Frederick i kāna palapala kauka kauka ma ka electrochemistry o nā mea radioactive i ka makahiki 1930. Ma mua iki aku, ua kau mua ʻo ia i kāna mau makemake i ka noiʻi ʻana o kāna wahine, a ma hope o ka pale ʻana i kā Frederick dissertation doctoral, ua hana pū lāua. ʻO kekahi o kā lākou holomua nui mua ʻo ia ka hoʻomākaukau ʻana i ka polonium, kahi kumu ikaika o nā ʻāpana alpha, ʻo ia hoʻi. nuclei helium.(24ʻO ia). Ua hoʻomaka lākou mai kahi kūlana kūpono ʻole, no ka mea, ʻo Marie Curie ka mea i hoʻolako i kāna kaikamahine me ka hapa nui o ka polonium. Ua wehewehe ʻo Lew Kowarsky, ko lākou hoa hana ma hope, penei: ʻO Irena "he ʻenehana maikaʻi loa", "ua hana ʻo ia me ka nani a me ke akahele", "hoʻomaopopo loa ʻo ia i kāna hana." He "noʻonoʻo ʻoi aku ka nani o kāna kāne." "Ua hoʻokō maikaʻi kekahi i kekahi a ʻike lākou." Mai ka manaʻo o ka mōʻaukala o ka ʻepekema, ʻo ka mea hoihoi loa iā lākou he ʻelua makahiki: 1932-34.
Ua ʻaneʻane ʻike lākou i ka neutron
"Aneane" mea nui. Ua aʻo koke lākou e pili ana i kēia ʻoiaʻiʻo kaumaha. I ka makahiki 1930 ma Berlin, ʻelua mau Kelemania - Walter Bothe i Hubert Becker - Noʻonoʻo i ke ʻano o ka hana ʻana o nā ʻātoma māmā i ka wā i hoʻopaʻa ʻia me nā ʻāpana alpha. Paleka Beryllium (49Be) i ka wā i hoʻopaʻa ʻia me nā ʻāpana alpha i hoʻokuʻu ʻia i ka pāhawewe ikaika loa. Wahi a ka poʻe hoʻokolohua, ʻo kēia radiation ka ikaika electromagnetic radiation.
I kēia manawa, ua hana ʻo Irena lāua ʻo Frederick i ka pilikia. ʻO kā lākou kumu o nā ʻāpana alpha ka mea ikaika loa. Ua hoʻohana lākou i ke keʻena kapua e nānā i nā huahana hopena. I ka pau ʻana o Ianuali 1932, ua hoʻolaha lākou ma ka lehulehu ʻo nā kukuna gamma ka mea i hoʻopau i nā protons ikaika nui mai kahi mea i loaʻa ka hydrogen. ʻAʻole lākou i maopopo i ka mea i loko o ko lākou mau lima a me nā mea e hana nei.. Ma hope o ka heluhelu ʻana James Chadwick (3) ma Cambridge ua hoʻomaka koke ʻo ia e hana, me ka manaʻo ʻaʻole ʻo ia ka radiation gamma, akā ua wānana nā neutrons e Rutherford i kekahi mau makahiki ma mua. Ma hope o ke ʻano o nā hoʻokolohua, ua ʻike ʻo ia i ka nānā ʻana o ka neutron a ʻike ʻo ia ua like kona nui me ka proton. I ka lā 17 o Pepeluali i ka makahiki 1932, ua waiho ʻo ia i kahi leka i ka puke pai Nature i kapa ʻia ʻo "The Possible Existence of the Neutron."
He neutron maoli nō ia, ʻoiai ua manaʻo ʻo Chadwick ua hana ʻia kahi neutron me kahi proton a me kahi electron. I ka makahiki 1934 wale nō ʻo ia i hoʻomaopopo a hōʻoiaʻiʻo ai ʻo ka neutron he ʻāpana haʻahaʻa. Ua hāʻawi ʻia ʻo Chadwick i ka Nobel Prize in Physics i ka makahiki 1935. ʻOiai ka ʻike ʻana ua hala lākou i kahi ʻike nui, ua hoʻomau ka Joliot-Curies i kā lākou noiʻi ma kēia wahi. Ua ʻike lākou ua hoʻopuka kēia pane i nā kukuna gamma me nā neutrons, no laila ua kākau lākou i ka hopena nuklea:
, kahi o Ef ka ikehu o ka gamma-kuantum. Ua hana ʻia nā hoʻokolohua like me 919F.
Ua hala ka wehe hou ana
He mau mahina ma mua o ka loaʻa ʻana o ka positron, ua loaʻa iā Joliot-Curie nā kiʻi o, ma waena o nā mea ʻē aʻe, he ala curved, me he mea lā he electron, akā wili i ka ʻaoʻao ʻē aʻe o ka electron. Ua kiʻi ʻia nā kiʻi i loko o kahi keʻena noe i loaʻa i loko o kahi kahua magnetic. Ma muli o kēia, ua kamaʻilio ka kāne e pili ana i nā electrons e hele ana i ʻelua ʻaoʻao, mai ke kumu a i ke kumu. ʻO ka ʻoiaʻiʻo, ʻo nā mea pili i ke kuhikuhi "i ke kumu" he mau positrons, a i ʻole nā electrons maikaʻi e neʻe ana mai ke kumu.
I kēia manawa, ma ʻAmelika Hui Pū ʻIa i ka hopena o ke kauwela o 1932, Carl David Anderson (4), ke keiki a nā malihini mai Suedena, ua aʻo ʻo ia i nā kukuna cosmic i loko o ke keʻena ao ma lalo o ka mana o kahi kahua magnetic. Hele mai nā kukuna Cosmic i ka Honua mai waho. ʻO Anderson, i mea e maopopo ai ke kuhikuhi a me ka neʻe ʻana o nā ʻāpana, i loko o ke keʻena i hala i nā ʻāpana ma o kahi pā metala, kahi i nalowale ai kekahi o ka ikehu. Ma ka lā 2 o ʻAukake, ʻike ʻo ia i kahi alahele, kahi āna i manaʻo ai he electron maikaʻi.
Pono e hoʻomaopopo ʻia ua wānana mua ʻo Dirac i ke ʻano o ke ʻano o ia mea. Akā naʻe, ʻaʻole i hahai ʻo Anderson i nā loina theoretical i kāna aʻo ʻana i nā kukuna cosmic. Ma kēia pōʻaiapili, ua kapa ʻo ia i kāna ʻike ʻana he pōʻino.
Eia hou, ua hoʻokau ʻo Joliot-Curie i kahi ʻoihana hiki ʻole ke hōʻole ʻia, akā ua hana hou i ka noiʻi ma kēia wahi. Ua ʻike lākou e hiki ke nalo nā kiʻi kiʻi gamma-ray kokoke i kahi nucleus kaumaha, e hana ana i ka lua electron-positron, ʻike ʻia e like me ke ʻano kumu kaulana a Einstein E = mc2 a me ke kānāwai o ka mālama ʻana i ka ikehu a me ka manawa. Ma hope mai, ua hōʻike ʻo Frederick iā ia iho aia kahi kaʻina o ka nalo ʻana o kahi pālua electron-positron, e hāʻawi ana i ʻelua gamma quanta. Ma waho aʻe o nā positrons mai nā pālua electron-positron, loaʻa iā lākou nā positrons mai nā hopena nuklea.
5. ʻEhiku Solvay Conference, 1933
Noho ma ka lālani mua: Irene Joliot-Curie (ʻelua mai ka hema),
Maria Skłodowska-Curie (ʻelima mai ka hema), Lise Meitner (ʻelua mai ka'ākau).
radioactivity hana
ʻAʻole i hana koke ka loaʻa ʻana o ka radioactivity artificial. I Feberuari 1933, ma ka pana ʻana i ka aluminika, ka fluorine, a me ka sodium me nā ʻāpana alpha, loaʻa iā Joliot nā neutrons a me nā isotopes ʻike ʻole. I Iulai 1933, hoʻolaha lākou, ma ka hoʻomālamalama ʻana i ka alumini me nā ʻāpana alpha, ʻike lākou ʻaʻole wale i nā neutrons, akā i nā positrons. Wahi a Irene lāua ʻo Frederick, ʻaʻole hiki ke hoʻokumu ʻia nā positrons i loko o kēia hopena nuklea ma muli o ka hoʻokumu ʻana o nā pālua electron-positron, akā pono e hele mai mai ka nucleus atomic.
Ua mālamaʻia kaʻEhiku Solvay Conference (5) ma Brussels maʻOkakopa 22-29, 1933. Ua kapaʻiaʻo "The Structure and Properties of Atomic Nuclei". Ua hele ʻia e nā physicists 41, me nā poʻe loea kaulana loa i kēia kahua ma ka honua. Ua hōʻike ʻo Joliot i nā hopena o kā lākou mau hoʻokolohua, e haʻi ana i ka hoʻomālamalama ʻana i ka boron a me ka alumini me nā kukui alpha e hoʻopuka i kahi neutron me kahi positron a i ʻole proton.. Ma keia ahaolelo ʻO Lisa Meitner Ua ʻōlelo ʻo ia ma nā hoʻokolohua like me ka alumini a me ka fluorine, ʻaʻole i loaʻa iā ia ka hopena like. I ka wehewehe ʻana, ʻaʻole ia i kaʻana like i ka manaʻo o nā kāne mai Palisa e pili ana i ke ʻano nuklea o ke kumu o nā positrons. Eia naʻe, i kona hoʻi ʻana i ka hana ma Berlin, ua hana hou ʻo ia i kēia mau hoʻokolohua a ma Nowemapa 18, ma kahi leka iā Joliot-Curie, ua ʻae ʻo ia i kēia manawa, i kona manaʻo, ʻike maoli ʻia nā positrons mai ka nucleus.
Eia kekahi, kēia ʻaha kūkā Francis Perrin, ko lakou hoa a me ko lakou hoaloha maikai mai Palika mai, i hai mai i ke kumuhana o positrons. Mai nā hoʻokolohua ua ʻike ʻia ua loaʻa iā lākou kahi kikoʻī mau o nā positrons, e like me ke ʻano o nā ʻāpana beta i ka palaho radioactive kūlohelohe. ʻO ka loiloi hou aʻe o ka ikaika o nā positrons a me nā neutrons Ua hele mai ʻo Perrin i ka hopena e hoʻokaʻawale ʻia nā hoʻokuʻu ʻelua ma ʻaneʻi: ʻo ka mua, ka hoʻokuʻu ʻana o nā neutrons, i hui pū ʻia me ka hoʻokumu ʻana o kahi nucleus unstable, a laila ka hoʻokuʻu ʻana o nā positrons mai kēia nucleus.
Ma hope o ka ʻaha kūkā ʻo Joliot i hoʻōki i kēia mau hoʻokolohua no ʻelua mahina. A laila, i Dekemaba 1933, hoʻopuka ʻo Perrin i kona manaʻo no ia mea. I ka manawa like, i Dekemaba Enrico Fermi manaʻo ʻia ka manaʻo o ka palaho beta. Ua lilo kēia i kumu kumu no ka wehewehe ʻana i nā ʻike. I ka hoʻomaka ʻana o ka makahiki 1934, ua hoʻomaka hou nā kāne mai ke kapikala Farani i kā lākou hoʻokolohua.
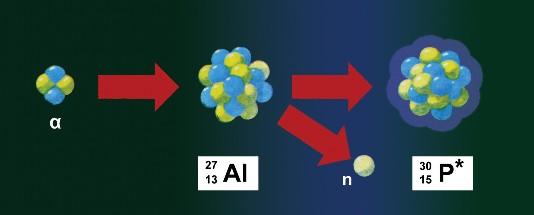
Ma ka lā 11 o Ianuali, ka ʻauinalā Poʻahā, ua lawe ʻo Frédéric Joliot i ka pepa alumini a hoʻopaʻa ʻia me nā ʻāpana alpha no 10 mau minuke. No ka manawa mua, ua hoʻohana ʻo ia i kahi counter Geiger-Muller no ka ʻike ʻana, ʻaʻole i ke keʻena noe, e like me ka wā ma mua. Ua ʻike ʻo ia me ka haʻohaʻo i kona wehe ʻana i ke kumu o nā ʻāpana alpha mai ka foil, ʻaʻole i pau ka helu ʻana o nā positrons, ua hoʻomau nā helu helu iā lākou, akā ua emi nui ko lākou helu. Ua hoʻoholo ʻo ia i ka hapalua o ke ola he 3 mau minuke a me 15 kekona. A laila ua hoʻemi ʻo ia i ka ikehu o nā ʻāpana alpha e hāʻule ana ma luna o ke kīwī ma ke kau ʻana i kahi pahu alakaʻi ma ko lākou ala. A ua liʻiliʻi nā positrons, akā ʻaʻole i loli ka hapalua o ke ola.
A laila ua hoʻokau ʻo ia i ka boron a me ka magnesium i nā hoʻokolohua like, a loaʻa i ka hapalua o nā ola i kēia mau hoʻokolohua o 14 mau minuke a me 2,5 mau minuke. Ma hope mai, ua hana ʻia kēlā mau hoʻokolohua me ka hydrogen, lithium, carbon, beryllium, nitrogen, oxygen, fluorine, sodium, calcium, nickel and silver - akā ʻaʻole ʻo ia i ʻike i kahi ʻano like me ka alumini, boron a me ka magnesium. ʻAʻole ʻokoʻa ka counter Geiger-Muller ma waena o nā mea i hoʻopiʻi ʻia a maikaʻi ʻole, no laila ua hōʻoia pū ʻo Frédéric Joliot e pili ana i nā electrons maikaʻi. He mea koʻikoʻi nō hoʻi ka ʻaoʻao ʻenehana i kēia hoʻokolohua, ʻo ia hoʻi, ʻo ka loaʻa ʻana o kahi kumu ikaika o nā ʻāpana alpha a me ka hoʻohana ʻana i kahi pākuʻi pākuʻi koʻikoʻi, e like me Geiger-Muller counter.
E like me ka mea i wehewehe mua ʻia e ka Joliot-Curie pair, hoʻokuʻu ʻia nā positrons a me nā neutrons i ka manawa like i ka hoʻololi nuklea i ʻike ʻia. I kēia manawa, ma hope o nā manaʻo a Francis Perrin a me ka heluhelu ʻana i nā manaʻo o Fermi, ua hoʻoholo lāua ua hoʻopuka ka hopena nuklea mua i kahi nucleus paʻa ʻole a me kahi neutron, a ukali ʻia e ka beta a me ka palaho o kēlā nucleus paʻa ʻole. No laila hiki iā lākou ke kākau i nā pane penei:
Ua ʻike nā Joliots i ka hopena o nā isotopes radioactive he pōkole loa ka hapalua o ke ola i ke ʻano. Ua hoʻolaha lākou i kā lākou hopena ma Ianuali 15, 1934, ma kahi ʻatikala i kapa ʻia ʻo "A New Type of Radioactivity". I ka hoʻomaka ʻana o Pepeluali, ua kūleʻa lākou i ka ʻike ʻana i ka phosphorus a me ka nitrogen mai nā hopena mua ʻelua mai nā mea liʻiliʻi i hōʻiliʻili ʻia. ʻAʻole i liʻuliʻu, ua wānana ʻia e hiki ke hana hou ʻia nā isotopes radioactive i nā hopena hoʻomake nuklea, me ke kōkua pū ʻana o nā protons, deuterons a me nā neutrons. I Malaki, ua hoʻoholo ʻo Enrico Fermi e hoʻokō koke ʻia kēlā mau hopena me ka hoʻohana ʻana i nā neutrons. Ua lanakila koke ʻo ia i ka pili.
Ua hāʻawi ʻia ʻo Irena lāua ʻo Frederick i ka makana no ka Nobel in Chemistry i ka makahiki 1935 no ka "synthesis of new radioactive elements". Ua wehe kēia ʻike i ke ala no ka hana ʻana i nā isotopes radioactive artificially, i loaʻa i nā noi nui a waiwai nui i ka noiʻi kumu, ka lāʻau lapaʻau, a me ka ʻoihana.
ʻO ka mea hope loa, pono e haʻi aku i nā physicists mai USA, Ernest Lawrence me nā hoa hana mai Berkeley a me nā mea noiʻi mai Pasadena, a ma waena o lākou he Pole e noho ana ma kahi hoʻolālā. Andrei Sultan. Ua ʻike ʻia ka helu ʻana o nā puʻupuʻu e nā helu helu, ʻoiai ua pau ka hana a ka accelerator. ʻAʻole lākou makemake i kēia helu. Akā naʻe, ʻaʻole lākou i ʻike e pili ana lākou i kahi mea hou koʻikoʻi a ua nele lākou i ka loaʻa ʻana o ka radioactivity artificial ...
